Statins Suck.
And why preventing your body from making the most important building block is a bad idea.
If you’ve been reading this Substack or following me on Twitter, you would know that I am not a fan of artificially reducing cholesterol. I am especially not a fan of statins - a class of cholesterol-lowering medication.
When they were first released, statins were hailed as the second coming - an integral part of any risk reduction strategy as it relates to cardiovascular disease.
After the implementation of new penal regulations for clinical trial reporting in 2004, most contemporary data has shed uncertainty on the benefits of taking statins.
This uncertainty has paved the way for research which takes a more critical view of this class of drugs. Its continued failure to meet expectations set by fraudulent pharma-funded research has also provided the population an opportunity to question the cholesterol-basis of heart disease in its entirety.
However, the inertia of the “cholesterol bad” and “statins good” mindset continues to this day.
No matter how much research I present pointing out the physiologically irrational nature of this approach, patients and physicians alike continue to point to statistical sleight-of-hand and treatment apologia.
In this article, I will address these rebuttals and in the process provide an alternative framework for understanding cholesterol and the effects of statins.
High Cholesterol Is “Bad”
I often hear something like this in response to criticism of statins.
“Ok, statins are bad. But, how am I supposed to get my LDL-cholesterol down?”
This is a great starting point, because it is where many stumble - physicians included.
First, cholesterol or sterols are the building-block of all life:
They make up the structure of our cells, the cellular organelles, the messaging infrastructure, the insulation for our neurons, the hormones our bodies use to regulate all function, and more. We would not be alive without cholesterol.
“Ok, but high cholesterol can be bad for you, right?”
Since sterol-derived compounds are essential to life, our body has a complex and fine-tuned method of regulating its presence in our bloodstream (really, this is what we are concerned about - blood serum cholesterol).
The real question is WHY?
Why is your serum cholesterol high?
The truth is, this is multi-factorial.
For most people in the developed world, cholesterol is elevated because it is responding to inflammatory stress, tissue damage or some other cause of elevated cell turnover.
This should come as no surprise. Look at the rates of obesity, lung disease, diabetes, strokes and heart attacks, and overall mitochondrial (i.e. metabolic) dysfunction.
Can you blame your body for pumping out more cholesterol? You need it to repair and heal!
Which means, if you want to have lower cholesterol - first, stop harming your body.
Now, there is also an increasing minority of the population who have another reason for their cholesterol to be high. Particularly lean and physically active people who are on very low carb diets like ketogenic and carnivore diets.
For these people with limited stores of fat, and low carbohydrate consumption…cholesterol and lipoprotein packages are a primary source of energy. Thus, the body maintains a higher amount in these healthier and more active people.
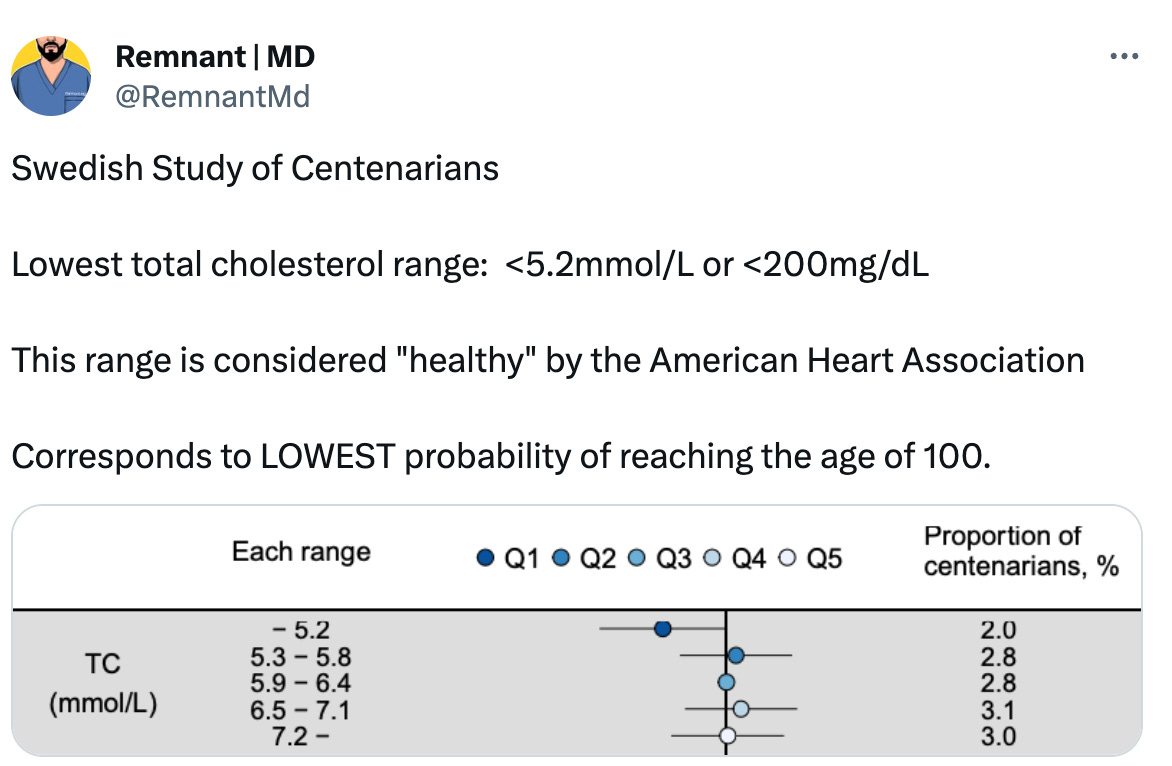
The implication is even more striking in the healthiest and most long-living people on Earth. Most recently, a Swedish study reported biomarkers from its citizens who managed to reach the age of 100 (centenarians) after decades of observational data.
What did they find?
In conclusion: there is no reason to assume that high cholesterol is bad.
“Ok, fine. Cholesterol is not bad. But, what about LDL cholesterol?”
Along similar lines of reasoning as the above, we will address LDL-cholesterol - which doctors still preach as “bad” cholesterol.
The first thing you need to know is that high LDL does not kill more than low LDL.

According to the American Heart Association, optimal LDL is less than 100 mg/dL. Strange isn’t it? Because, the graph above (which controls for other risk factors) clearly demonstrates higher all-cause mortality in people with LDL lower than 120 mg/dL. More importantly, as you increase LDL-cholesterol above 180 mg/dL there is no significant rise in risk of all-cause mortality.
Similar to what we discussed above, the body will churn out high LDL in response to inflammatory stress and tissue damage.
This is characterized most strikingly in patients who are critically-ill, ie. in the intensive-care unit (ICU). Over the course of several decades, we have observed repeatedly (1, 2, 3) this strange relationship. ICU patients with low cholesterol and low LDL have the highest risk of short-term complications and death.
To phrase this another way, those in critical condition have a higher chance of survival if their LDL cholesterol is high.
Why? Again, our body pumps out cholesterol based on need for tissue repair and regeneration.
How do you think the body will achieve this if they are on a statin?
How Statins are “Good”
Now that we’ve cleared the air about cholesterol, we move onto statins.
First of all, I will admit this:
Insofar as statins are capable of suppressing immune function/inflammation - they can be of short-term value.
In recent years, several studies have shown that statins provide some benefit to very ill patients because of the effect they have on inflammation. There are a couple of mechanism by which they can have this effect. For example, statins inhibit the function of our immune cells (as they do to all of our cells), and they inhibit the function of mitochondria such that the process which consumes the most energy (inflammation and repair) is blunted.
The American Heart Association has published several papers to this effect. In one study using FDG-PET metabolic imaging, they demonstrate that statins provide benefit to patients with high-risk coronary artery plaques by altering its metabolic activity. This is important because, high-risk plaques are those with the most intense inflammatory change. To the best of our current understanding, the inflammatory and biomechanical stress on arterial plaque is by far the most important predictor of its stability. Unstable plaques are highly likely to rupture and clog-up.
Additionally, by the same mechanism, statins can prevent the progression of soft-plaque. As we will see, this comes at costs to both the body and the arteries.
As far as the benefits of statins go, that is it. This one short-term morbidity benefit.
The only other explicitly claimed benefit is: low LDL/low cholesterol. This assumes that high cholesterol (as defined by the AHA) is inherently bad in and of itself. But, we have already established that this isn’t the case.
Why Statins Suck.
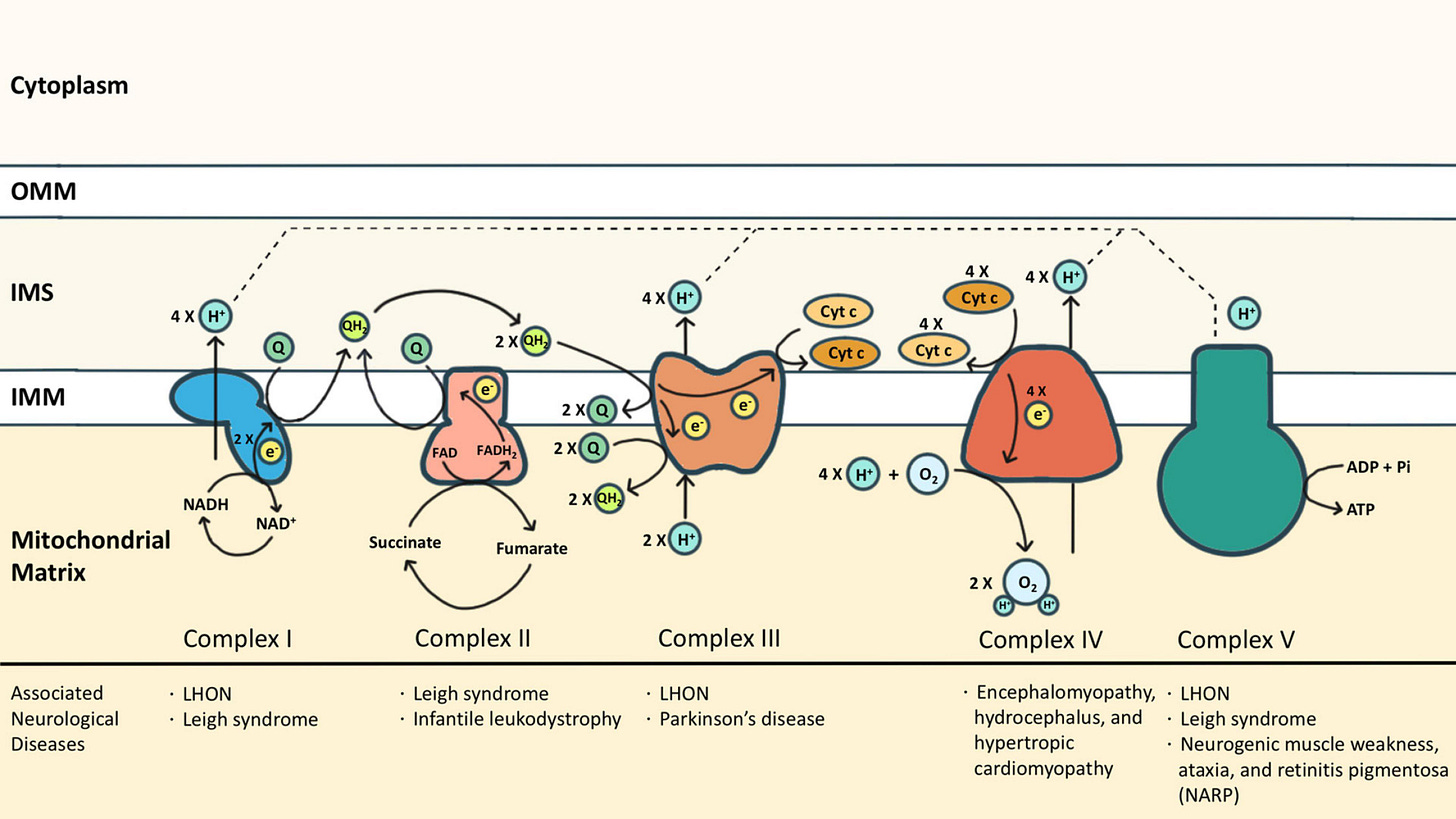
Really, there are two important biosynthetic pathways to understand to appreciate the harm caused by Statins.
Figure 1, the Mevalonate pathway:
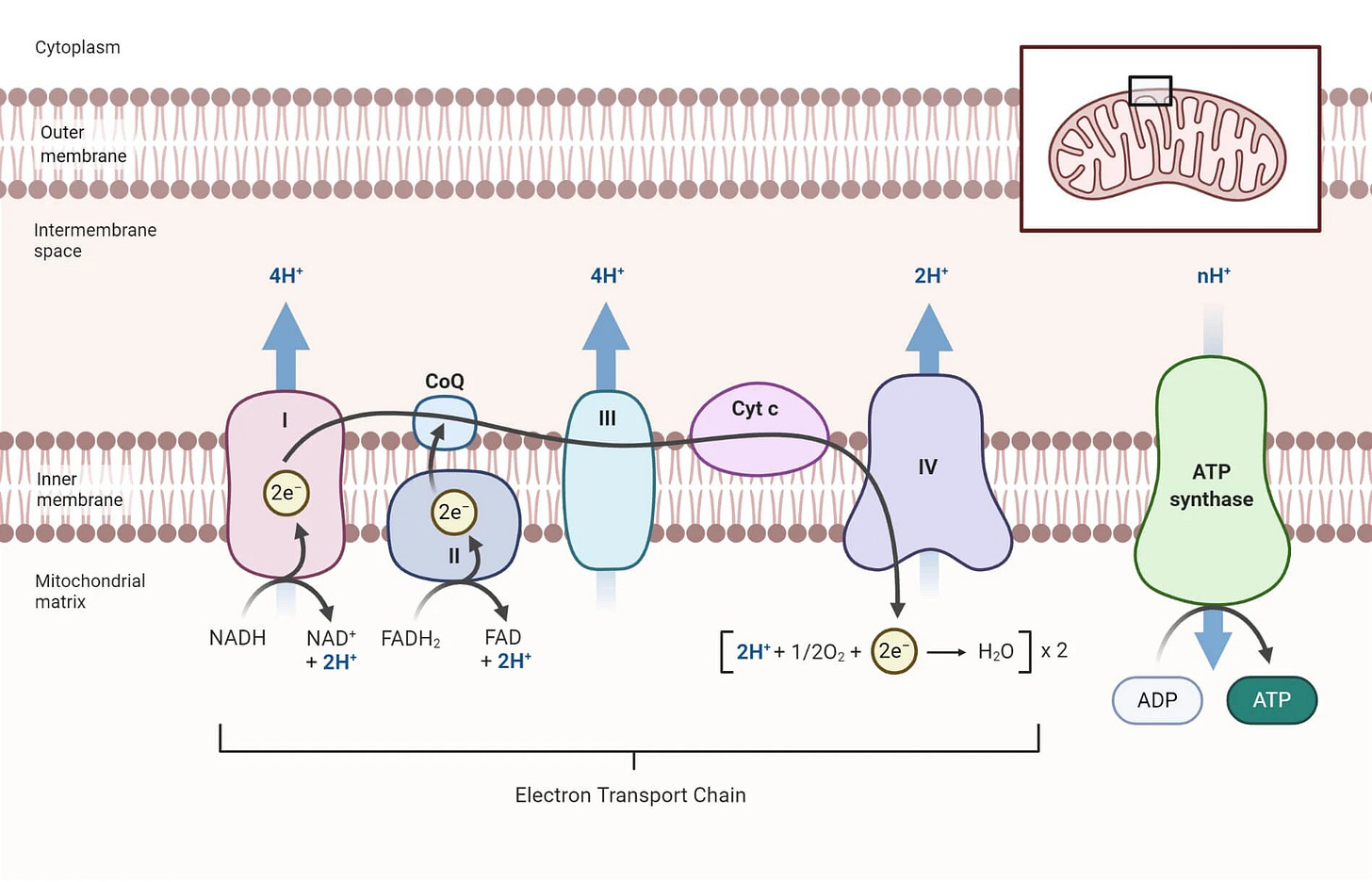
Figure 2, the electron transport chain:
Remember I told you that cholesterol and sterol-derived molecules make up our entire being? Figure 1 demonstrates the relevant pathway.
Statins are also known as HMG-CoA Reductase Inhibitors. This enzyme (HMG-CoA Reductase) catalyzes the production of mevalonate - the precursor to thousands upon thousands of molecules that are integral to normal body function.
Statins do not merely reduce cholesterol. They prevent the synthesis of countless molecules critical to our cells. Vitamin K, Ubiquitin, Heme A, Phosphatidylcholine, Farnesyl Pyrophosphate (FPP) and Geranylgeranyl Pyrophosphate, and so much more.
It is so disruptive to our cellular machinery, in any other world it would be classified as chemotherapy.
And wouldn’t you know it? Several new studies are demonstrating the mechanism by which statins can be used to kill cancer cells:
“But, my doctor tells me the only side-effect to be worried about is muscle pain.”
Yes, muscle pain is by far the most reported side-effect of taking statins. The reason for this are as follows:
Muscle pain is the easiest side effect you can feel.
For example, you cannot feel the onset of diabetes. That doesn’t mean statins don’t cause new-onset diabetes. They do.
Muscles are highly energy-demanding. Which brings us to Figure 2.
As you can see from the diagram of the electron transport chain (inside the mitochondria), there are several membrane-associated proteins which generate the electro-chemical gradient necessary to make ATP (energy currency of the cell).
Statins block the production of Coenzyme Q10 and Heme A. Heme A is the functional component of Cytochrome C Oxidase. Thus, statins block two critical proteins for the production of energy needed to meet the demands of our body.
One of the few places we can feel the effects of this are in our muscle. Many people also report something like a “brain fog” when taking statins. This is also, in large part, due to the energy imbalance statins cause. Because, our brain is the other highly energy-dependent organ.
In fact, there are many ways that statins contribute to cognitive dysfunction and dementia by its impact on the electron-transport chain.
Inhibition of the pathway in Figure 1 and Figure 2 also contribute to an interesting observation across long-term statin users, especially as it relates to coronary artery disease. There has been the strange observation that statin use is associated with worsening calcification of the coronary arteries. Statin apologists will argue that this is a good thing! Because hardened arteries are less likely to rupture. Which is true.
But, this phenomenon is an indicator of a bigger problem:
Blockage of HMG-CoA Reductase prevents the production of Vitamin K2
this vitamin is integral to proper regulation of calcium.
in its absence, we inappropriately deposit calcium in soft-tissue.
Not just in coronary arteries.
Blockage of the electron-transport chain prevents the formation of new water.
Decreased body water production is a hallmark of aging. This water is normally produced in the mitochondria.
The net effect is dehydration and stiffening of the related soft-tissue.
Taken together, these systemic problems contribute to stiffening and calcification of coronary arteries observed in statin users.
But, this isn’t necessarily a good thing. Stiffening of arteries is yet another hallmark of aging. The downside is that our vessels are less compliant to changes in demand for organ perfusion, as well as increased pressure due to higher output from the heart. This comes with consequences of its own.
Further, what’s important to appreciate is that this effect is not limited to the coronaries! It will happen wherever there are mitochondria and a need for cholesterol.
I could go on, but this rant is already getting long.
Suffice it to say, at this point I wouldn’t even prescribe a statin to my enemy.









My Medicare Advantage HMO plan mandates two exams per year, which I call the "Are you still alive?" visits. My cholesterol is always a bit north of 200, which causes my doctor great angst. This in spite of the fact that I am probably one of very few "old" patients she has (I am 76) that have no prescriptions, no metabolic diseases, and start each day with 100 push-ups and a 5k walk. Dr. Robert Lustig says that the triglyceride to HDL ratio is a predictor of heart disease, and a value of 1.5 means "you will live forever". I'm at 1.6, and proceeding on the assumption that I have ¼ of my life yet to be lived. I'm a firm believer in Dr. Peter Attia's concept of "Medicine 3.0" in his book 'Outlive', which is based on your root cause approach.
"LDL levels can be elevated in individuals with low thyroid activity because T3 helps sensitize the LDL receptor on the cell membrane. Lower active T3 will cause an inability of the LDL particles to dock on the cell membrane drop the cholesterol and fat soluble nutrients into the cell. So the body adapts and increases the amount of overall LDL cholesterol similar to how insulin goes up in response to insulin resistance." -- Dr David Jockers