Phytic Acid & Mineral Deficiencies
Plants resort to substances that are toxic to their predators as a defense. If you include plants in your diet - which you likely do - then you have certainly been exposed to phytic acid.

Note: This article was drafted by my guest writer and brother, Saint Keto, and edited by yours truly. If you found this informative, do give @SaintKeto a follow on Twitter.
Background
Plants, just like animals, do not wish to be eaten. Due to their relative inability to physically protect themselves, they resort to substances that are toxic to their predators. If you include plants in your diet - which you likely do - then you have certainly been exposed to phytic acid.
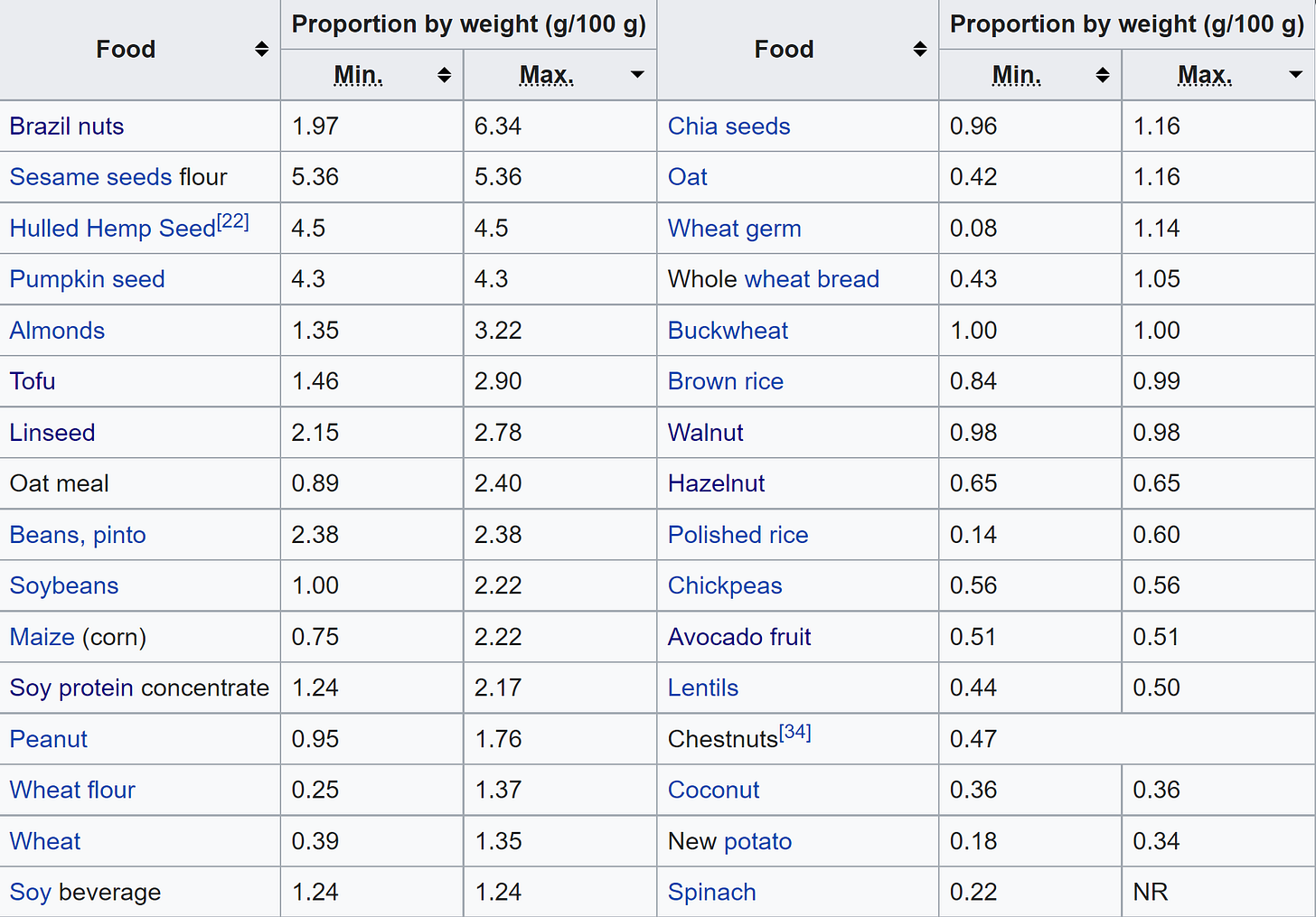
Phytic acid or phytate is a common ingredient in many staple foods - including legumes, grains, seeds, cereals, and other vegetables. As innocent as these foods may seem, they can cause some pernicious nutritional deficiencies. If you are low in minerals such as zinc, iron, or calcium, phytic acid could possibly be the culprit.
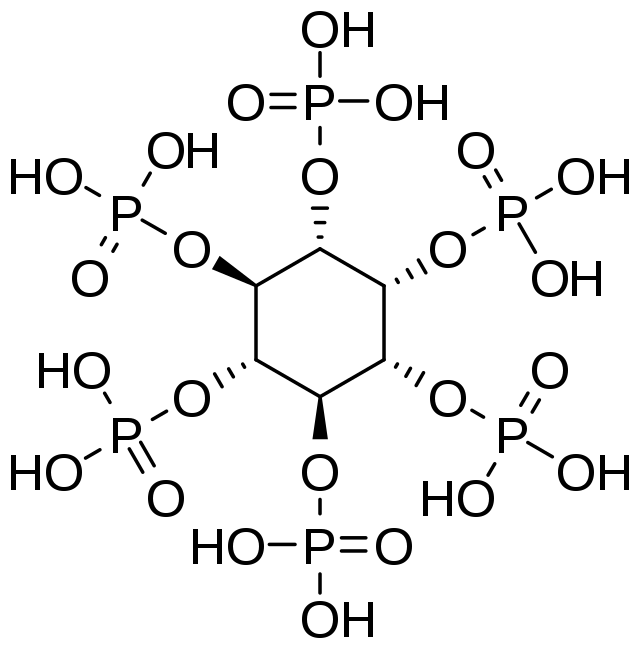
Phytic acid is an ester of inositol and contains 6 dihydrogenphosphate [H2PO4-] ions. The phytate anion is caused by the partial ionization of the phosphate groups at a certain pH level. Any number of the phosphates in phytic acid can be partially reduced at physiological pH.
Mineral Imbalances
Phytic acid was discovered in the early 1900s. Soon after, reports suggesting the detriment of phytic acid were published when pigs and rats on a high-phytate diet were observed to have rickets - an infantile vitamin D deficiency which causes soft bones.
It is believed that cereal in the diet reduced available calcium, resulting in soft bones. In 1940, it was found that phytate inhibited calcium absorption.
A high phytate diet was also found to have an inhibitory effect on zinc, when a low zinc bioavailability was observed in chicks. The adverse effect on human zinc absorption was reported in 1973 (Reinhold et al).
Soon after, studies on different plant foods were conducted to investigate zinc absorption. Different extraction rates of wheat bread supported the idea of zinc malabsorption. Soy protein was found responsible for zinc deficiency in a study where adolescent girls consumed soy protein as a substitute for meat protein. Furthermore, soy-formula (high in phytate) has been found to inhibit zinc absorption more than milk-based formula.
The Golden Ratio...of Phytate to Zinc
A study of phytate vs zinc molar doses in diets conducted in 2002 generated some reinforcing results. The phytate-to-zinc molar ratios ranged from 0.03:1 to 28.6:1, and it was ultimately concluded that a significant hindrance to zinc absorption was induced by a 5.7:1 or higher ratio of phytate-to-zinc. A similar ratio of 6:1 was found to lower absorption when soy protein was used in a 1984 study.
In mice, it has been shown that a 10-to-1 ratio significantly impairs zinc absorption, though mice have some phytase - an enzyme that breaks down phytate - which they can use to their benefit. Phytase is also found to some extent in plants, though it does not offset the negative effects of phytate as much.
Molar ratios of [Phytate x Calcium]/[Zinc] have also been suggested (Lönnerdal et al) as a predictor of zinc bioavailability. This follows from the idea that more phytate can bind to calcium ions in the duodenum, allowing for less zinc to get complexed with phytate and consequently more zinc to be available for absorption. Phytate has a strong binding affinity to minerals such as zinc, calcium, iron, etc.
More than Zinc
Other than zinc, phytate inhibits calcium, iron, and other minerals like copper, magnesium, and manganese - some others to a smaller extent.
Multiple studies suggest that phytate has a strong binding affinity and inhibitory effect on iron. When phytic acid from soy protein isolate was reduced from 4.9-8.4 mg/g to less than 0.01 mg/g, iron absorption increased at least fourfold (Hurrell et al). Even small amounts of phytate prove inhibitory for iron - and another study suggested that complete dephytinization of cereal increased iron absorption twelvefold.
Furthermore, it has been observed that adding phytase from the fungus Aspergillus niger to a grain-containing diet significantly increases iron absorption - from 14% to 26% in wheat bran with inactivated phytase, for example.
One study done on rats showed that as phytate increased in their controlled diet, magnesium absorption declined. Starting at the control group, 43.5% of the ingested magnesium was absorbed. For each new protocol arm, 4 grams/kg of sodium phytate was added until they stopped at 20 grams/kg. The uptake fell to 36.9%, 33.5%, 30.2%, 27.0%, and 27.6%, respectively. A human study also found a negative correlation between phytic acid and magnesium.
Copper deficiency may also be attributed to high phytate content, though this remains uncertain. In rats, copper retention might have been impaired due to high phytate, though it is possible that zinc had an effect - as zinc and copper are competitive. The phytate-copper complex is generally soluble, which likely renders the threat of phytate insignificant.
For manganese, the results vary and the range of effect is also unknown. Its binding affinity to phytate is lower than those of zinc/iron/copper and it is homeostatically regulated through bile excretion, which eases the balance.
Link between Phytic Acid & Inositol
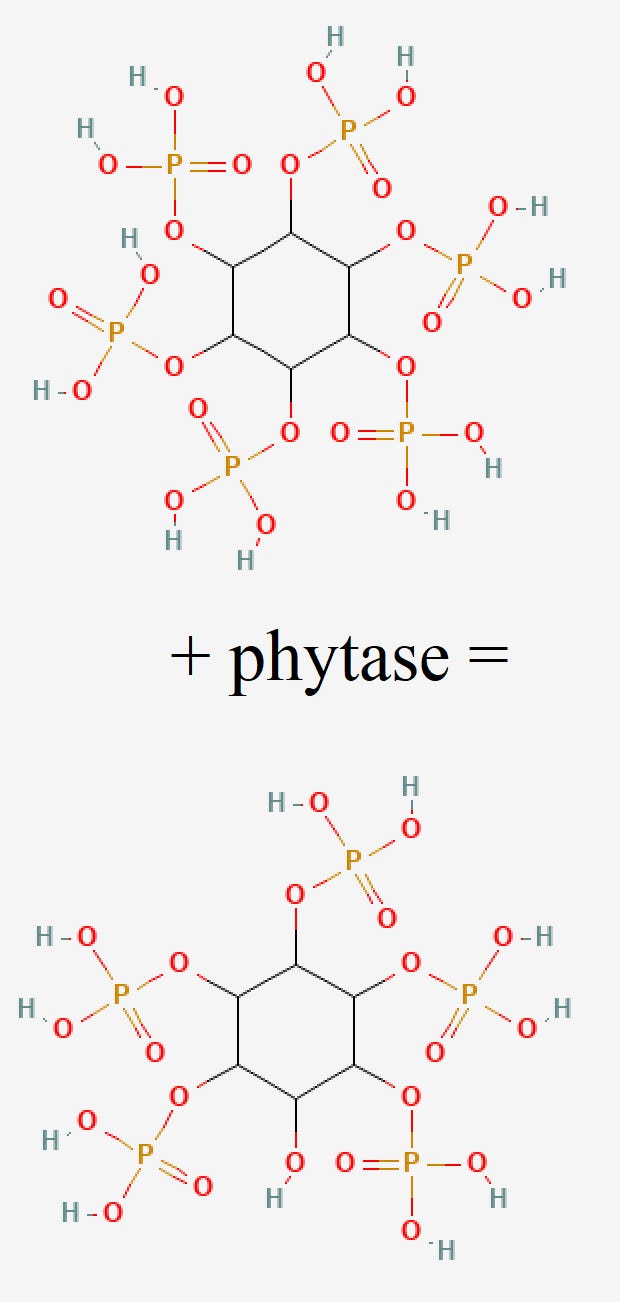
So, how does phytase neutralize the negative effects of phytic acid?
Remember when we noted that phytic acid is composed of inositol and six dihydrogenphosphates? Well, for better description, we can refer to another chemical name of phytate - inositol hexakiphosphate. Phytase is designed to target the individual phosphate groups - its substrate is the DHP.
Phytase dephosphorylates (removes a phosphate group) inositol hexakiphosphate (IP6) in order to produce inositol pentakiphosphate (IP5). At every incremental dephosphorylation, the molecule is reduced to tetra (IP4), tri (IP3), and bi (IP2) -phosphates. Each of the different stages of dephosphorylation has a different effect on mineral bioavailability.
A study on suckling rat pups in 1989 indicated that IP6 and IP5 have a strong inhibitory effect on zinc absorption, while those of IP4 and IP3 were virtually insignificant. Another study analyzed the effects of the same phytate derivatives on zinc and calcium uptake by percent, and similar results were found.
Radioactive isotopes were used in experimentation. Zinc-65 uptake in the liver after 6 hours of ingestion was 5% for IP6, 19% for IP5, 28% for IP4, 29% for IP3, and 31% for zinc (II) chloride, without any added inositol phosphate (Lönnerdal et al).
For the calcium experiment, the rat pups were given calcium-45 (also radioactive) along with phytate derivatives. The amount of unabsorbed calcium was 17% for IP6, 1.4% for IP5, 0.5% for both IP4 and IP3, and 0.5% in the control group without any phytate derivative.
Although these two studies were not conducted on humans, they demonstrate how antagonistic phytate/IP5 are on zinc and calcium absorption, and thus, how effective phytase is. However, humans have thirty times less phytase activity than that of rats, so we need to look for different ways of ridding phytate.
By now, you must be thinking 'how do I get rid of this stuff'?
Don’t worry, there are both enzymatic and non-enzymatic ways of minimizing phytate content from plants. Preparation techniques like steaming, baking, fermenting, sprouting, hydrothermal processing, and malting can be effective for reducing phytate in plants. Adding exogenous phytase is also an option.
In one study, cereals such as wheat, barley, rye, and oats were germinated (sprouted) to measure endogenous phytase activity (Bartnik et al). The hydrolysis of phytate was listed in ascending order: oats < wheat/barley < rye. This is likely due to the higher content of endogenous phytase in wheat/barley/rye, and inactive phytase in oats.
Inactive phytase = more phytic acid in the diet
Germination in itself does not cause a huge decrease in phytate, but malting can be effective. Another study found that malting each of the aforementioned grains - by grinding and soaking them for 2 hours - decreased their phytate content by 95%, while it took relatively longer with oats (Sandberg et al).
Malting of breakfast cereals alone was found to increase zinc absorption from 15% to 23%.
Hydrothermal processing of barley porridge also increased zinc bioavailability, from 11% to 25% (phytate content was reduced fourfold).
Baking bread with yeast also significantly reduces phytate content, from 66-97% - the most pronounced effect was in sourdough bread (Fröhlich et al).
Health Consequences of Phytate-induced Mineral Deficiency
Do we really need all these metals that phytate seems to reduce the absorption of?
Well, yes.
A zinc deficiency is responsible for a wide array of problems including stunted growth, immune and cognitive issues, poor pregnancy outcomes, low testosterone, prostate issues, and many more. Zinc is also an important cofactor for many enzymes.
Low iron causes deficiency of a vital component of red blood cells called heme - a component of hemoglobin. Heme is responsible for transporting oxygen in your body, and it requires an iron atom to function properly. Without the iron, it carries much less oxygen and leads to iron deficiency anemia. Symptoms include shortness of breath, fatigue, weakness, chest pain, cold and pale skin, etc. Just think about what low oxygen can do to you.
In a prior INSIGHTS Series article, we discuss the health consequences of low magnesium:
Though low copper does not have much of a link with phytate, it is worth mentioning that copper plays a role in the production and functioning of collagen, and a deficiency can be responsible for arthritis, fatigue, osteoporosis, brittle bones, joint pain, hair loss, inflammation, and the like.
Phytic Acid in the Marketplace
Here is an example of a company who has taken steps against the detrimental effects of phytic acid in their product.
You may have heard of or tried Huel - a meal replacement product which comes in the form of bar, liquid, grains, or powder.
From one of their products, Huel Black Edition, it looks like the manufacturers are cognizant of the detriment of phytic acid:
Source: https://huel.com/pages/the-huel-black-edition-formula-explained
It is commendable that the company has taken steps to improve mineral quality of their product, though it is worth noting that the fortified iron and zinc may or may not be optimally bioavailable - one additional reason being that zinc and iron compete for absorption and thus it is not ideal to take much of both at the same time.
Further Reading
https://ifst.onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2621.2002.00640.x
https://academic.oup.com/jn/article-abstract/119/2/211/4738155
https://www.sciencedirect.com/science/article/abs/pii/S0271531798000852
https://academic.oup.com/ajcn/article-abstract/56/3/573/4715420
https://econtent.hogrefe.com/doi/abs/10.1024/0300-9831.74.6.445